- 1Department of Otorhinolaryngology, Head and Neck Surgery, University Hospital Leipzig, Leipzig, Germany
- 2Department of Radiation Oncology, University Hospital Leipzig, Leipzig, Germany
- 3Department of Nuclear Medicine, University Hospital Leipzig, Leipzig, Germany
- 4Department of Pathology, University Hospital Leipzig, Leipzig, Germany
- 5Department of Internal Medicine II, Division of Oncology, University Cancer Center Leipzig (UCCL), Leipzig University Medicine, Leipzig, Germany
Background: About five to 10% of cancers in the head and neck region are neck squamous cell carcinoma of unknown primary (NSCCUP). Their diagnosis and treatment are challenging given the risk of missing occult tumors and potential relapse. Recently, we described human papillomavirus (HPV)-related NSCCUP-patients (NSCCUP-P) as a subgroup with superior survival. However, standardized diagnostic workup, novel diagnostic procedures, decision-making in the multidisciplinary tumor board (MDTB) and multimodal therapy including surgery and post-operative radio-chemotherapy (PORCT) may also improve survival.
Methods: For assessing the impact of standardized diagnostic processes simultaneously established with the MDTB on outcome, we split our sample of 115 NSCCUP-P into two cohorts treated with curative intent from 1988 to 2006 (cohort 1; n = 53) and 2007 to 2018 (cohort 2; n = 62). We compared diagnostic processes and utilized treatment modalities applying Chi-square tests, and outcome by Kaplan–Meier plots and Cox regression.
Results: In cohort 2, the standardized processes (regular use of [18F]-FDG-PET-CT imaging followed by examination under anesthesia, EUA, bilateral tonsillectomy and neck dissection, ND, at least of the affected site) improved detection of primaries (P = 0.026) mostly located in the oropharynx (P = 0.001). From 66.0 to 87.1% increased ND frequency (P = 0.007) increased the detection of extracapsular extension of neck nodes (ECE+) forcing risk factor-adapted treatment by increased utilization of cisplatin-based PORCT that improved 5-years progression-free and overall survival from 60.4 and 45.3 to 67.7% (P = 0.411) and 66.1% (P = 0.025).
Conclusions: Standardized diagnostic workup followed by ND and risk-factor adapted therapy improves survival of NSCCUP-P.
Introduction
The earliest description of cervical lymph node metastasis as the primal symptom of cancer by Hayes Martin dates back to 1944 (1). Today a subgroup of about 5% of head and neck cancer cases (2) and 3–5% of all human cancer cases (3) are diagnosed based on a lump in the neck (4) without obvious signs for a primary tumor and are designated neck squamous cell carcinoma of unknown primary (NSCCUP). Identification of occult primary tumors is challenging, as small flat lesions in anatomical complex structures of the oropharynx, oral cavity, hypopharynx and larynx may be invisible and impalpable (5, 6). Therefore, and as NCCN and ASCO guidelines recommend (7, 8), clinical examinations shall be accompanied by radiological imaging using ultrasound sonography and computed tomography (CT) or magnet-resonance imaging (MRI) upfront excision biopsies of potentially affected regions, as well as examination under anesthesia (EUA) involving bilateral tonsillectomy (9).
New imaging technologies combining [18F]-FDG positron-emission tomography (PET) and CT (PET-CT) or MRI (PET-MRI) facilitate detection of occult tumors and metastasis (10, 11). Therefore, ASCO recommends PET-CT/PET-MRI imaging followed by EUA and adapted treatment planning (8). However, to the best of our knowledge, literature showing facilitated diagnostic processes, decision-making for particular treatment leading to improved survival of NSCCUP-patients (NSCCUP-P) based on full implementation of these guidelines (7, 8) does not exist.
We established a multidisciplinary tumor board (MDTB) for discussing each individual case aiming on consented recommendations for the evidence-based probably best treatment option. We implemented all now recommended diagnostic procedures and risk factor-adapted treatment already in 2007. By comparing outcome in our cohort of NSCCUP-P before and after implementation of the MDTB and standardization of workup including risk factor-adapted treatment, we show the benefit from this sound approach.
Materials and Methods
Study Population and Patient Samples
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the University Leipzig (votes 201-10-12072010 and 202-10-12072010). Patients diagnosed at our clinic between 1988 and 2018 with a lump in the neck without clinical sign of a primary tumor (ICD-10-C77 or ICD-10-C80) are included and underwent diverse diagnostics until establishing the MDTB and standardizing the diagnostic workflow. Finally, we analyzed 115 pathological confirmed NSCCUP-P out of 272 cases in two cohorts (Figure 1).

Figure 1 CONSORT diagram showing the selection criteria of neck squamous cell carcinoma of unknown primary (NSCCUP) patients of the two cohorts before and after standardization of diagnostic workup and therapy as well as the distribution of primary tumors detected leading to exclusion of initially suspect NSCCUP patients.
Clinical Work-Up for NSCCUP-P
Clinical work-up for NSCCUP-P until 2006 (cohort 1) varied and included e.g. clinical examination, ultrasound sonography and other variable procedures (Table 1) before treatment. Since 2007 (cohort 2) clinical work-up was standardized and included, as recommended (7, 8), clinical examination, ultrasound sonography, contrast-enhanced CT, PET-CT/PET-MRI followed by EUA accompanied by taking multiple biopsies from the epipharynx, base of the tongue or lingual tonsillectomy plus bilateral tonsillectomy. Patient and tumor characteristics, diagnostic procedures, treatment and clinical follow-up were recorded in our Microsoft Access® tumor database (TDB) and OncoFlow® (12–14) (Tables 1–3).
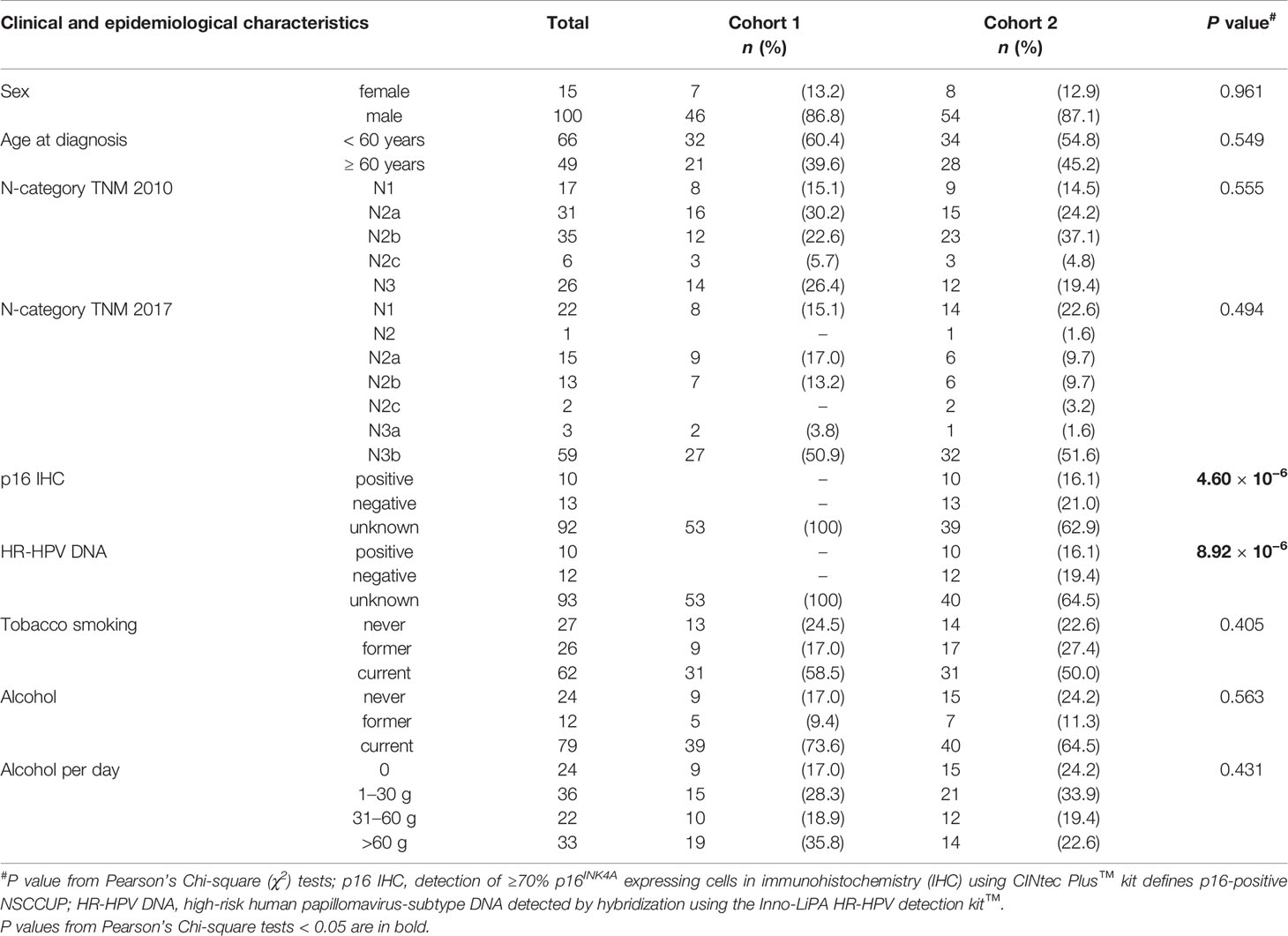
Table 1 Clinical and epidemiological characteristics, diagnostic procedures and treatment of neck squamous cell carcinoma of unknown primary (NSCCUP) patients of cohorts 1 and 2.
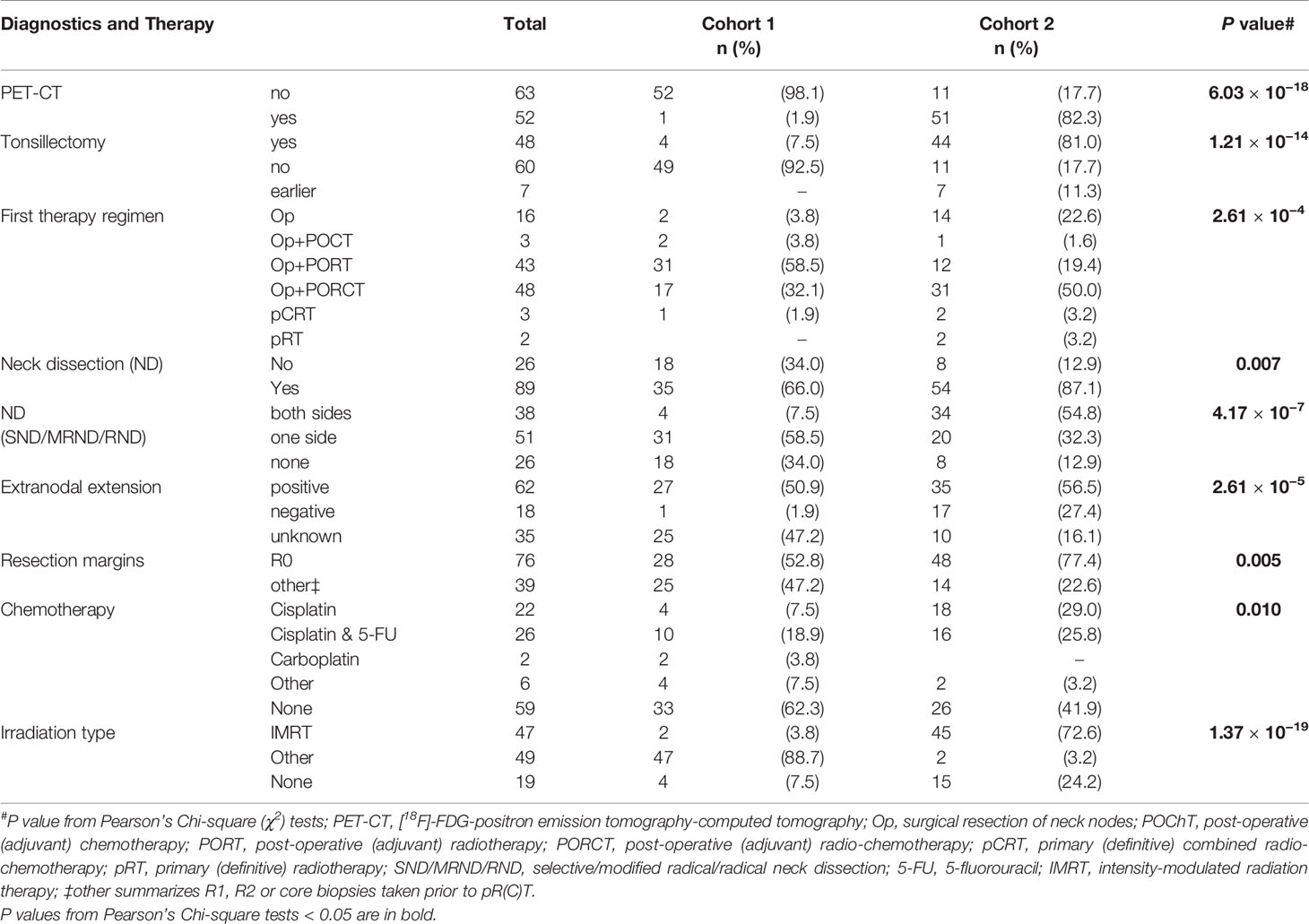
Table 2 Diagnostic procedures and treatment regimens applied to neck squamous cell carcinoma of unknown primary (NSCCUP) patients of cohorts 1 and 2.
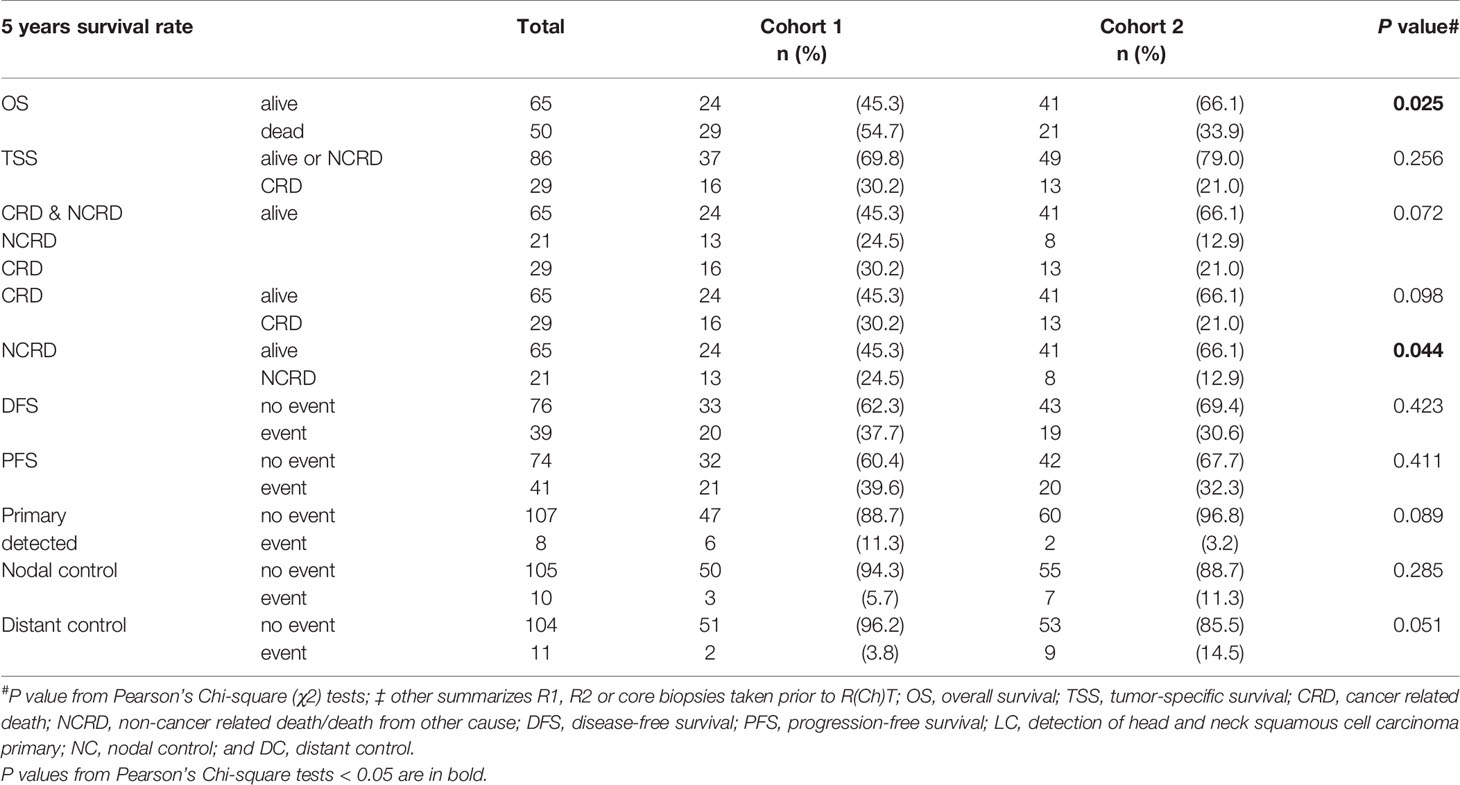
Table 3 Various survival measures for 5-years outcome of neck squamous cell carcinoma of unknown primary (NSCCUP) patients of cohorts 1 and 2.
CT and PET-CT Imaging
According to clinical guidelines, all patients received a thorax and a head and neck CT scan during staging. In 2006, a PET-CT became available. Beginning in 2007, all patients with clinical diagnosis of NSCCUP were scheduled to receive a [18F]-FDG-PET-CT scan (11, 15); however, 11 (17.7%) had no PET-CT imaging before biopsies were taken (Table 2).
PET-CTs were analyzed by an experienced board-certified nuclear-medicine physician and a radiologist. Sites of tumor involvement were identified visually by enhanced, non-physiologically [18F]-FDG uptake.
Decision-Making Process in the MDTB
The decision-making process in the MDTB followed ASCO and NCCN guidelines (7, 8) and principles published earlier (12–14, 16–18). Briefly, radiologist and nuclear medicine specialist presented all radiological imaging. The MDTB consisting of head neck and maxillofacial surgeons, a pathologist, an oncologist, a radiation oncologist, and other clinical staff involved in the treatment of head and neck cancer patients discussed results of diagnostic procedures. Considering the general health and comorbidity of the patient the pre-therapeutic MDTB according to guidelines (7, 8) mostly recommended neck dissection as first treatment.
Histological Examination and HPV Status
All resected specimen underwent pathological examination. Pathological reports were available for all 115 patients. Besides hematoxylin–eosin (HE) staining, molecular analyses of p16 by immunohistochemistry utilizing the CINtec kit were routinely performed since 2013. A sub-cohort of patients participated in a study approved by our Ethics Committee (votes 201-10-12072010 and 202-10-12072010). Table 1 includes some data respective to HR-HPV published elsewhere (19).
Treatment Modalities
After ND and obtaining the pathology reporting evident risk factors (especially ECE+), the post-operative MDTB recommended mostly cisplatin-based post-operative chemo-radiotherapy (PORCT) according to guidelines (7, 8, 20). In cohort 2, NSCCUP-P with ND and detection of only unilateral N+ (N2b) without risk factors present (up to 2 N+ <6 cm, no ECE, R0/no incision biopsy) received PORT of 60 Gy ipsilateral and 50 Gy contralateral, independent from ND also of the unaffected site or not. Irradiation after resection of a single node without risk factors (<6 cm, no ECE, R0/no incision biopsy) was unilateral 60 Gy, whereas presence of risk factors for local recurrence (bilateral N+, N2c, or one node ≥6 cm, N3, or ECE+, R1) indicated the need for bilateral irradiation with 64 Gy and concomitant cisplatin 200 mg/m2. In cohort 2, according to our standardized protocol and dependent on affected lymph node levels, up to 50 Gy were applied prophylactic to the pharynx (including base of the tongue) and nasopharynx.
In majority, the NSCCUP-P agreed and received MDTB-recommended treatment. Only eight cohort-2 patients disagreed with the recommendation, refused PORT or PORCT, and had solely surgery (Table 2).
Statistical Analysis
Patient characteristics and follow-up data from TDB and OncoFlow® were analyzed in relation to clinical characteristics of patients, risk factors (daily alcohol consumption categorized in 0, ≤30 g, ≤60 g, >60 g; tobacco smoking categorized in ≤ or > 10 pack years; smoking status categorized in never, former, current smoker), diagnostic procedures used, treatment modalities, N and M categories, HPV status. Associations between categorical variables were examined by Pearson’s Chi-square test. We calculated overall survival (OS) time from date of diagnosis to date of death (event), or end of follow-up (censored); tumor-specific survival (TSS) time from date of diagnosis to date of cancer-related death (event) censoring other causes of death or end of follow-up. Time to non-cancer related death (NCRD) defined events as death from other cause censoring cancer-related death. Disease-free survival (DFS) measured time-interval from curative R0 resection or last fraction of irradiation to relapse or death (event) censoring patients alive with no sign of disease at last follow-up. We calculated progression-free survival (PFS) time from date of diagnosis to date of relapse or death (event), or end of follow-up without progression (censored). We defined nodal control (NC) as time from NSCCUP diagnosis to relapse focusing on detected disease-positive nodes independent from neck laterality, and measured distant control (DC) as time from NSCCUP diagnosis to distant metastasis detected. We additionally recorded any detection of a primary squamous cell carcinoma in the head and neck region (HNSCC detection). We used receiver-operating characteristic (ROC) curves to find optimum cut-off values for quantitative parameters to binary classify patients. We analyzed survival using the Kaplan–Meier method (21) applying log-rank tests (22) and hazard ratios (HR) using Cox proportional hazard models (23) utilizing the conditional logistic regression forward method, and bootstrapping (24) (SPSS version 24, IBM Corporation, Armonk, New York). We considered 2-sided P <0.05 significant.
Results
Patient Characteristics
The CONSORT diagram (Figure 1) shows eligible and excluded patients in cohorts 1 and 2, highlighting increased detection of primaries with standardized clinical workup (P = 0.026). The distribution in histologically defined entities and localization of SCC primary lesions detected also differed substantially (P = 0.006 and 0.001, respectively). Table 1 shows patients’ characteristics. Cohorts differed insignificantly regarding sex, history of consuming alcohol or nicotine and age at diagnosis, whereas applied diagnostic and treatment procedures differed significantly (Tables 1 and 2).
PET-CT Analyses
According to PET-CT availability until 2006, 1/53 (1.9%) NSCCUP-P of cohort 1 and 51/62 (82.3%) of cohort 2 underwent [18F]-FDG-PET-CT imaging as part of the pre-therapeutic staging (P = 6.03 × 10−18). All analyzed NSCCUP were detected positive for [18F]-FDG enrichment, whereas non-physiologically enhanced uptake by other organs was absent or led to diagnosis of the initially occult primary (Figure 1 and Table 2).
Tonsillectomy, Neck Dissection and Pathological Examination
According to our in-house standard operating procedure (SOP) defined for diagnostic and treatment of suspect NSCCUP-P in 2007, we exclusively performed surgery after completed staging and imaging and omitted wedge excisions as recommended (7, 8). Surgery then consisted of ND at least of the affected site combined with extended bilateral tonsillectomy, both performed more frequently (P = 0.007 and 1.21 × 10−14, respectively). This altogether led to improved identification of primary tumors, predominantly cancers of the tongue base and the palatine tonsils (Figure 1). Cohorts 1 and 2 differed significantly regarding the depth of clinical examination documented in the pathologic reports; ECE+ and, in case of ECE+, especially R0 resection with >5 mm distance to clear margins were more frequent in cohort 2 (Table 2).
Prevalence of HPV in NSCCUP
As previously shown in a separate analysis of 46/62 NSCCUP-P with tumor and blood samples available (cohort 2), we analyzed HPV DNA, HPV E6*I mRNA and p16INK4A as well as antibodies to HPV proteins in sera (19). We found 9/62 (14.5%) HPV-driven cases defined by positivity for p16INK4A together with presence of HR-HPV DNA and E6*I mRNA. One patient each was negative for either p16INK4A (1/10) or HR-HPV DNA and E6*I RNA (1/10; Table 1). Further 3 NSCCUP-P had no FFPE specimen available to analyze p16 and HPV and were defined as HPV-driven based on HR-HPV serostatus (19).
Treatment Modalities
The treatment of NSCCUP-P differed significantly between cohorts (Tables 2 and 3). Bilateral tonsillectomy and ND improved detection of primaries. ND, however, also led to increased detection of ECE in cohort 2.
For a singular SCC-positive node (N1 according to TNM 7th ed. without ECE), the MDTB recommended clinical follow-up, and therefore sole surgery without adjuvant treatment increased in cases without risk factors, whereas, based on risk-adapted decision-making, cisplatin-based Op+PORCT increased. Related to increased cisplatin usage from 14/53 (26.4%) to 34/62 (54.8%), chemotherapies increased overall (P = 0.01). After 2006, we predominantly (P = 1.37 × 10−19) applied intensity-modulated radiotherapy (IMRT). However, irradiation time, fractionation and nominal doses administered by fixed-field radiation (cohort 1) or IMRT (cohort 2) differed not significantly.
Survival in Cohorts 1 and 2
The median follow-up was 37.4 months in the total sample, and 49.6 and 32.9 months in cohorts 1 and 2 (P = 0.198). Outcome assessed at 2 years differed only insignificantly. In the total cohorts 1 versus 2, 2-years OS was 69.7% (95% CI 57.3–82.1%) versus 80.2% (95% CI 69.7–90.8%); 2-years TSS was 79.3% (95% CI 67.8–90.8%) versus 84.9% (95% CI 75.2–94.6%); 2-years DFS was 74.4% (95% CI 62.4–86.5%) versus 70.3% (95% CI 57.9–82.6%); 2-years loco-regional control 87.1% (95% CI 77.2–96.8%) versus 87.2% (95% CI 77.4–97%); 2-years DC was 98.1% (95% CI 94.5–100.0%) versus 85.8% (95% CI 76.7–95%). The 5-years OS in cohort 2 was improved (P = 0.025); other outcome measures did not demonstrate significant improvements at this time (Table 3). Kaplan–Meier plots of cumulative survival (Figure 2), however, show improved outcome in cohort 2 but without reaching statistical significance.
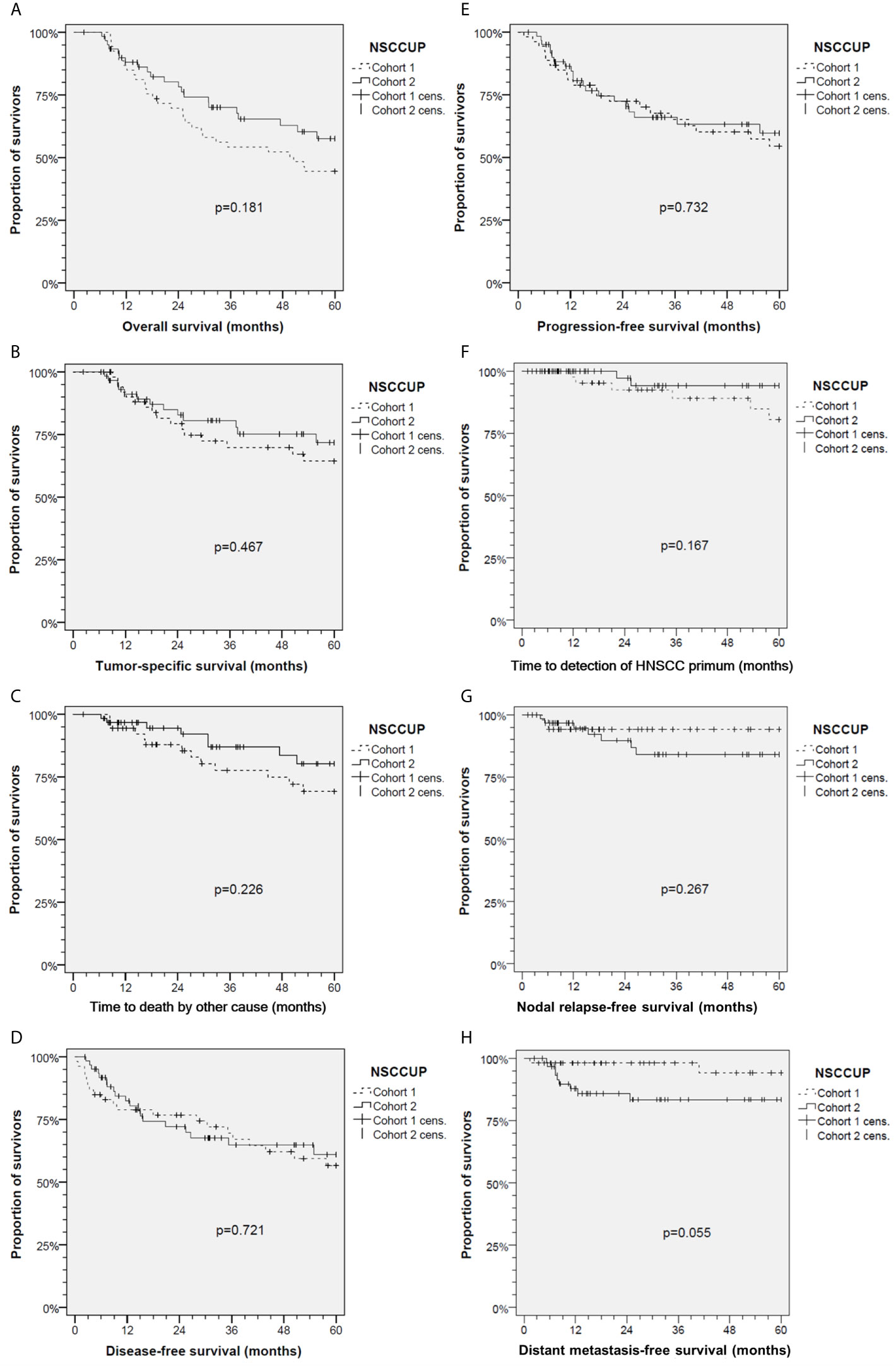
Figure 2 Kaplan–Meier cumulative survival analyses of neck squamous cell carcinoma of unknown primary (NSCCUP) patients of cohorts 1 and 2 for (A) overall survival; (B) tumor-specific survival; (C) survival according to non-cancer death/death from other cause; (D) disease-free survival; (E) progression-free survival; (F) Time to detection of head and neck squamous cell carcinoma primary; (G) Nodal relapse-free survival and (H) Distant metastasis-free survival. P values shown are from 2-sided log-rank tests.
Survival Related to Non-Standardized Versus Standardized Diagnostic Procedures
We analyzed a possible link between non-standardized versus standardized diagnostic procedures. Frequency of PFS and DFS events differed not significantly between cohorts; one patient from cohort 1 had a PFS event related to lung cancer of other histology (no DFS event). No particular diagnostic procedure alone improved outcome (all P > 0.2).
Survival Related to N category, ND and PORCT
The number of neck nodes removed and found positive for disease (N+) defined the N category linked to different outcome (Table S1). According to Kaplan–Meier curves, NSCCUP-P of cohorts 1 and 2 undergoing ND had identical OS and TSS.
The survival in NSCCUP without ND differed significantly regarding TSS (7/18 CRD), death by other cause (6/18 NCRD) and OS (13/18 deaths in total) in cohort 1 versus 0/8 in cohort 2 (P = 0.076, 0.117 and 0.018, respectively, in log-rank tests). The highest differences in OS and TSS relate to ND, detection of ECE, and risk factor adapted use of cisplatin-based regimens (Figures 3 and 4).
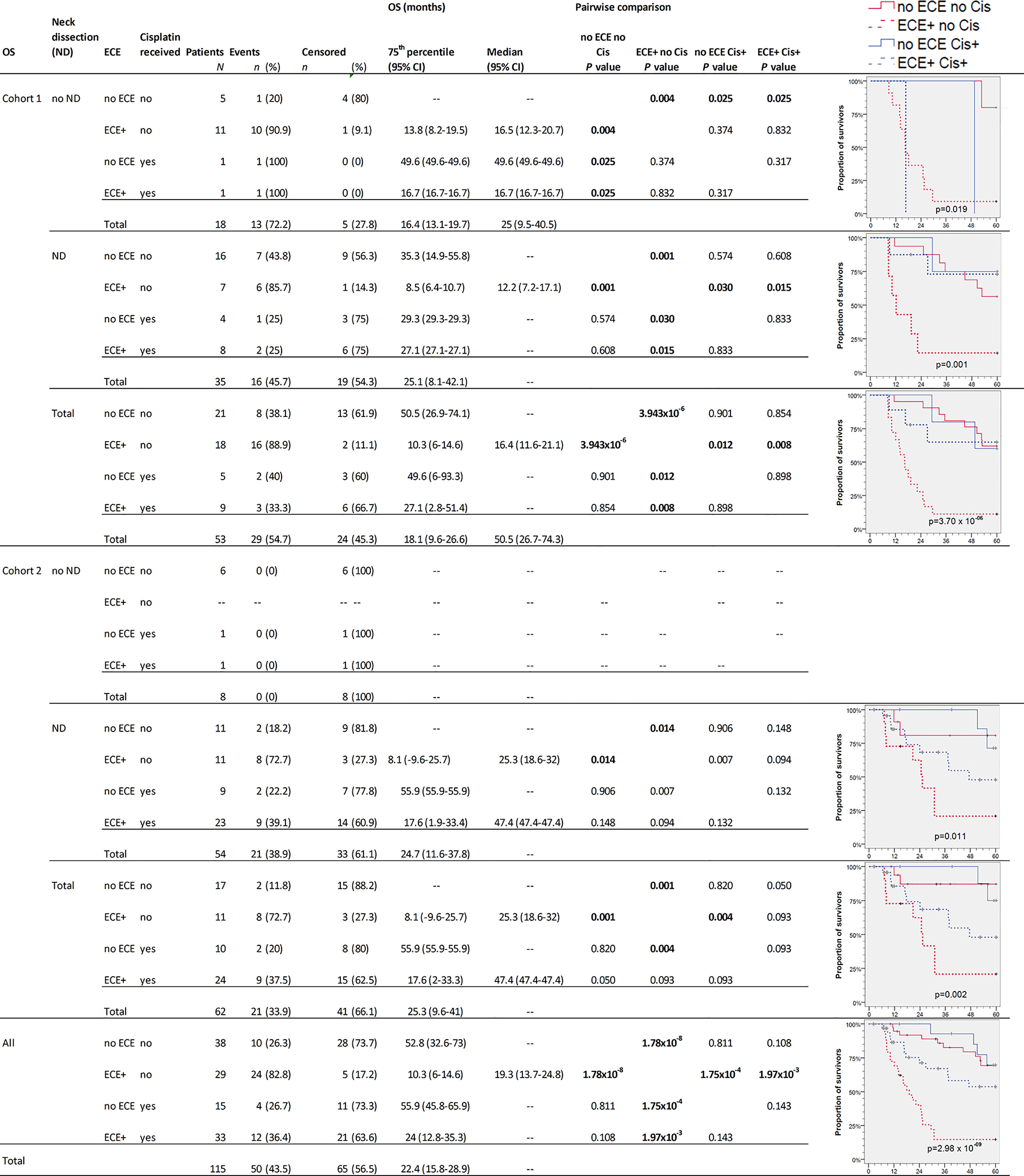
Figure 3 Kaplan–Meier cumulative survival (KM) plots for overall survival (OS) of squamous cell carcinoma of unknown primary patients (NSCCUP-P) demonstrate the improved outcome achieved by standardized treatment including neck dissection (ND) and cisplatin-based radio-chemotherapy (Cis+) for NSCCUP-P with extracapsular extension of neck nodes (ECE). Besides KM plots, the numbers of patients and events are shown with 75th percentile and median along with their respective 95% confidence interval (95% CI) together with P-values from pairwise comparisons in various strata combining ND, ECE and Cis in cohorts 1 and 2 as well as for all NSCCUP-P.
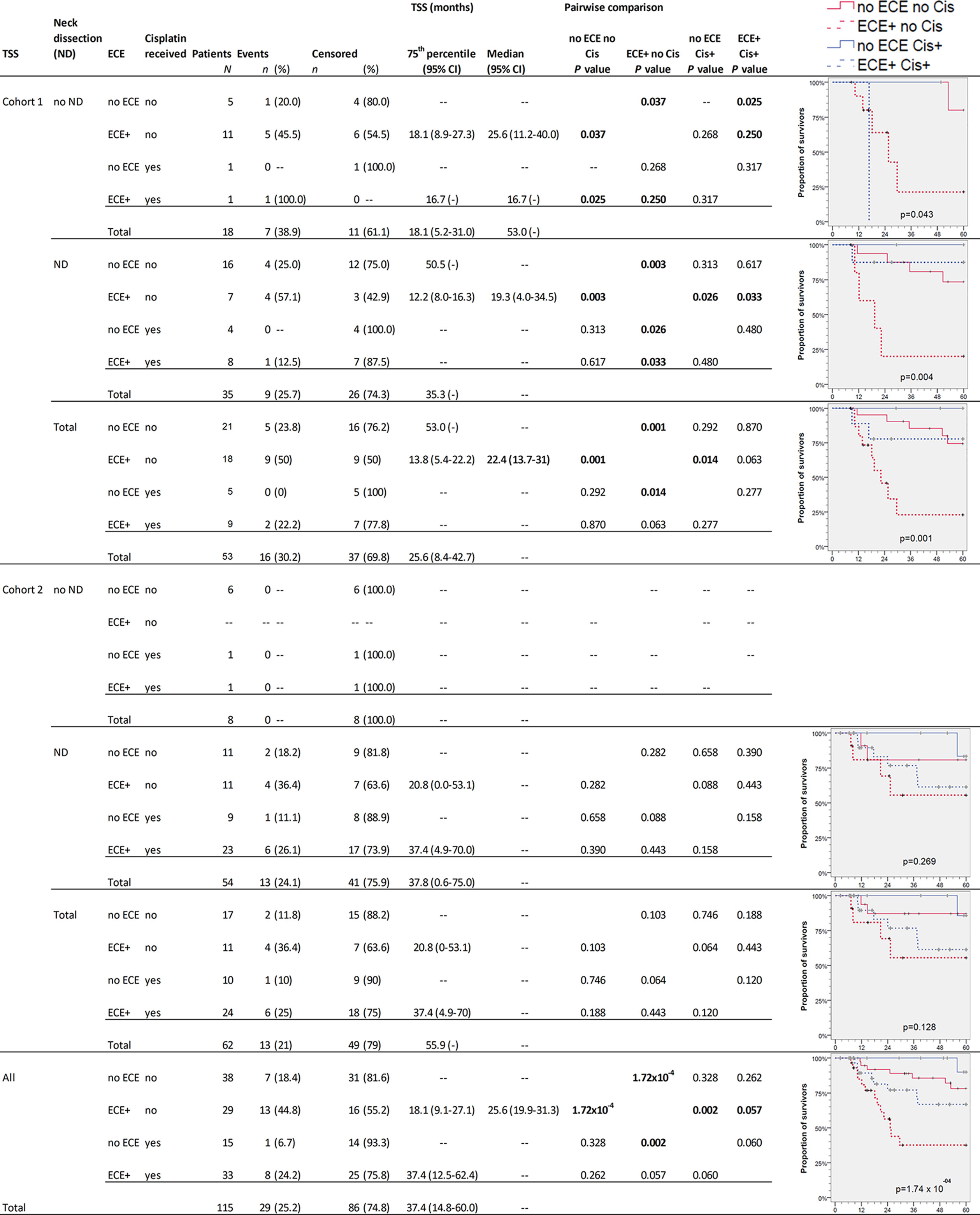
Figure 4 Kaplan–Meier cumulative survival (KM) plots for tumor-specific survival (TSS) of squamous cell carcinoma of unknown primary patients (NSCCUP-P) demonstrate the improved outcome achieved by standardized treatment including neck dissection (ND) and cisplatin-based radio-chemotherapy (Cis+) for NSCCUP-P with extracapsular extension of neck nodes (ECE). Besides KM plots, the numbers of patients and events are shown with 75th percentile and median along with their respective 95% confidence interval (95% CI) together with P-values from pairwise comparisons in various strata combining ND, ECE and Cis in cohorts 1 and 2 as well as for all NSCCUP-P.
We detected a significantly improved outcome (P = 2.98 × 10−9 and 1.74 × 10−4 for OS and TSS, respectively) of patients receiving cisplatin-based Op+PORCT. Those patients who had ECE but did not receive cisplatin-based Op+PORCT experienced worst outcome with median OS and TSS of 19.3 (95% CI 13.7, 24.8) and 25.6 (95% CI 19.9, 31.3) months only. Significant best outcome had patients without ECE (Figures 3 and 4). Comparing PORT and cisplatin-based PORCT after ND of NSCCUP without ECE of both cohorts combined, we detected numerical improved TSS according to 6 cancer-related deaths in 27 NSCCUP-P after PORT (22.2%) versus 1 cancer-related death (7.7%) in 13 NSCCUP-P receiving PORCT (P = 0.257). However, the corresponding Kaplan–Meier plots and log-rank test confirmed that the TSS difference between PORT and PORCT was not significant (P = 0.266).
Survival Related to HPV
As shown previously, HPV-related NSCCUP-P demonstrated significant superior OS and PFS in univariate analyses (19) therefore they are not presented here again.
Multivariate Cox Regression Models for Outcome
Figure 5 shows forest plots, HR, 95% CI, and the corresponding P values of covariates in the multivariate Cox proportional hazard model achieving highest significance for the respective outcome measure. As recommended (24), we show p values from bootstrapping utilizing 1,000 iterations for independent predictors (Pi). This internal validation revealed significance of all models and confirmed all Pi being significant with only one exception: The (adverse) effect of surgery on TSS that lost significance applying the bootstrap. Thus, we identified the appearance of surgery as designated Pi as an artifact of the statistical model because surgery improves NC and DC. On the opposite, both types of event, NC events (nodal failure) and DC events (distant failure) led to impaired TSS.
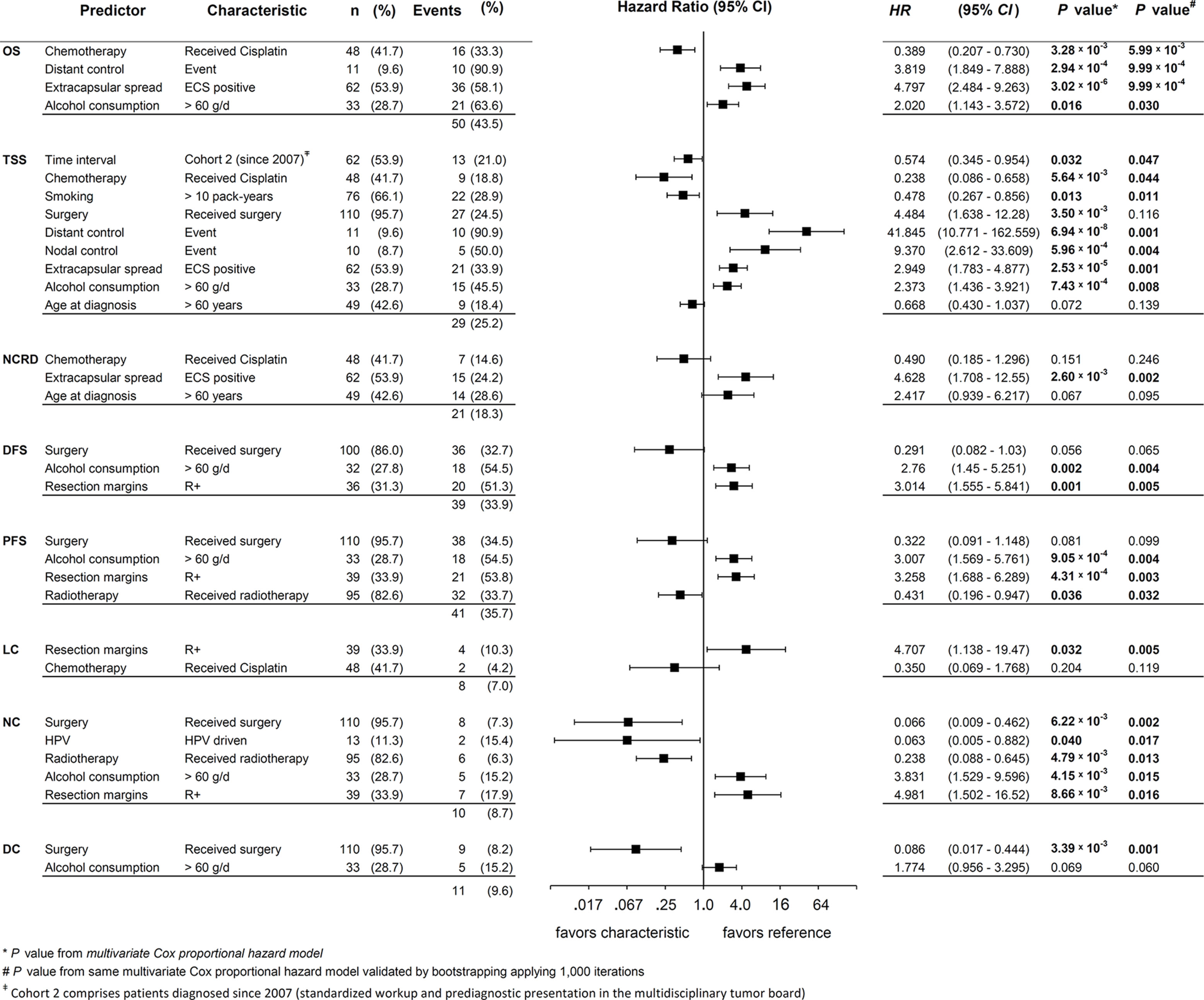
Figure 5 Forest plots for hazard ratio (HR) and 2-sided 95% confidence interval (95% CI) from multivariate Cox proportional hazard models for various outcome measures and survival of neck squamous cell carcinoma of unknown primary (NSCCUP) patients built using the stepwise forward likelihood ratio method. Events represents the numbers of events found for the individual predictor and (%) the percentage of patients experiencing the event among those with the characteristic, and HR, 2-sided 95% CI, and 2-sided P value* the outcome attributable to this characteristic according to the final model for the particular measure. P values# are 2-sided P values from internal validation using bootstrapping applying 1,000 iterations. P values of independent predictors < 0.05 are in bold.
Consequently, nodal and distant failure are linked to treatment without surgery (compare the opposite direction of effects in multivariate analyses including DC and NC exerted by surgery on TSS versus the effects of surgery on DC and NC; Figure 5). Related to confounding by factors also linked to improved outcome in the total NSCCUP cohort, multivariate analyses did not confirm any significant impact of HPV-driven disease on survival. With NC being the only exception, the close correlation of HPV status and predominant Pi demonstrates that HPV-relatedness is rather a confounder linked to many independent predictors of improved survival without itself being an independent predictor of improved outcome (Figure 5).
We identified the standardized diagnostic workup and decision-making for surgery followed by risk-factor adapted adjuvant therapy applied to NSCCUP-P since 2007 as a significant Pi for improved TSS.
Discussion
Our retrospective study demonstrates improved outcome in NSCCUP achieved through evidence-based decision-making by a MDTB for the probably best treatment option for the individual NSCCUP-P based on standardized diagnostic workup including [18F]-FDG-PET-CT imaging, bilateral tonsillectomy and neck dissection. Our MDTB comprises all professions involved in the clinical workup of head and neck cancer patients (12). Pre- and post-surgery presentation of each NSCCUP case in the MDTB along with SOPs in diagnostics and treatment mirror the recently published guidelines (7, 8).
To overcome variable preoperative workup, we implemented a standardized diagnostic approach including contrast-enhanced CT and PET-CT imaging followed by EUA with random biopsies taken from the base of the tongue or lingual tonsillectomy, biopsies from the epipharynx followed by bilateral tonsillectomy and ND. The increased detection of occult tumors demonstrates that this standardized workup is effective. The diagnosis of occult tumors was facilitated (Figure 1), and the number of primary SCC detected at various head and neck sites increased substantially (P = 0.001). Shortly after establishing comprehensive use of PET-CT for CUP patients in our clinic in 2007, the prospective DAHANCA-13-study showed changes in therapeutic strategy in about 25% of NSCCUP-P after diagnosis of the primary tumor using PET-CT in diagnostic workup und also recommended use of FDG-PET for diagnostic processes in NSCCUP-P (25). Our results are in line with their findings (Figure 1). The high rate of subclinical (occult) primary lesions detected by PET-CT and EUA confirms recent results from a meta-analysis demonstrating the highly increased probability to detect small lesions given combined utilization of these modern approaches (26). Mostly, the primary tumors identified in our cohort were located in the base of tongue or palatine tonsils, in line with recent data (27, 28). At time of NSCCUP diagnosis and more than 6 months thereafter a total of six (five within 60 months) occult HNSCC tumors were detected in our cohort 1 but only two in cohort 2 (compare Table 1 and Figure 2F).
Besides improved outcome in the oropharyngeal cancers presenting initially as NSCCUP (data not shown), we provide evidence that a standardized diagnostic workup followed by risk-adapted treatment described improves the outcome even in definitive NSCCUP-P without detection of primary lesions (Table 1 and Figures 2–5). The evidence-based decision-making for further diagnostic and the standardized treatment planning for CRT, Op + PORT or risk-adapted cisplatin-based Op + PORCT improve outcome as demonstrated in oropharyngeal cancer (29). The diagnostic step carrying a substantial therapeutic effect improving survival was ND. Kaplan–Meier curves and 75th percentiles highlight comparable OS (27.1 versus 24.7 months) and TSS (35.3 versus 37.4 months) of NSCCUP-P undergoing ND in both cohorts (Figures 3, 4 and Table S1). In contrast, OS in NSCCUP-P without ND differed significantly with 13/18 (72%) deaths in cohort 1 (CRD: 7/18, NCRD: 6/18) versus 0/8 in cohort 2 (P = 0.076, 0.117, and 0.018, respectively). None of the 18 cohort 1-patients without ND underwent tonsillectomy; 8/18 (44.5%) experienced wedge excision and 10/18 (55.5%) node extirpation only. The eight NSCCUP-P of cohort 2 without ND had extended tonsillectomy in 7/8 (87.5%) and extirpation of their singular neck node in 6/8 (75%) cases. Those two NSCCUP-P of cohort 2 without ND or node extirpation received curative IMRT applying cisplatin-based CRT and definitive RT, respectively. The latter age-79 male with high comorbidity is disease-free now for more than 38 months.
Strikingly, N categories according to TNM 2017 (8th edition) proofed to be superior regarding prognostication of survival especially when considering results of p16 immunohistochemistry (leading to down-staging of p16+ NSCCUP) and ECE (up-staging of p16-negative NSCCUP with ECE+). Increased ECE detection, however, was dependent on a multitude of effective changes facilitating in-depth pathological examination in NSCCUP-P of cohort 2.
Given the heterogeneity of NSCCUP and the small case numbers compared, the general survival benefit for the total cohort 2 was below statistical significance (according to log-rank tests) (26).
However, the subgroup analyses presented for OS (Figure 3) and TSS (Figure 4) demonstrate that multimodal therapy combining upfront surgery (ND and tonsillectomy) followed by cisplatin-based PORCT are able to overcome the prior significant worse outcome of ECE+ NSCCUP and hence is strongly recommended. ECE is discussed in various retrospective studies whether (30) or not (31) being a prognostic factor for poorer survival. While demonstrating the negative impact of ECE on OS, TSS, and NCRD and being a significant Pi for impaired survival even after internal validation by bootstrapping, we showed importance of considering ECE in decision making for risk-factor adapted therapy and favoring cisplatin-based chemotherapy to overcome the increased risk of dying (Figures 3 and 4). In cohort 2, the increased utilization of cisplatin-based PORCT led to improved survival and reduced the difference between NSCCUP-P with and without ECE. Whenever cisplatin is used in risk-adapted adjuvant treatment for ECE+ detected in resected nodes, differences diminished to insignificance. Therefore it is no surprise that NSCCUP-P of cohort 1 undergoing ND followed by cisplatin-based PORCT had the same survival as those in cohort 2 (Figures 3 and 4). This finding hence is compatible with a significant negative impact of ECE+ in a cohort with low frequent (29%) use of PORCT (30) comparable to our cohort 1 (26.4%) that also showed significant impaired survival in ECE+ patients, whereas use of cisplatin-based PORCT in cohort 2 was 54.8% and linked to improved survival. This benefit in TSS and OS in patients receiving cisplatin-based PORCT that could not be shown in rather small NSCCUP-P studies before (30, 32) confirms findings in advanced head and neck cancers (16, 17, 20). Whereas ND followed by cisplatin-based PORCT should be recommended to NSCCUP-P with ECE+, it is not yet completely clear if the numerical benefits regarding OS and especially TSS in NSCCUP without ECE seen in our study outweigh the potential harms from cisplatin-based PORCT. A randomized controlled trial addressing this question by analyzing outcome including health-related quality of life would allow to obtain the required evidence.
In multivariate analyses, the most stable covariates affecting various survival measures were alcohol consumption >60 g/d, ECE+ and positive resection margins (R+), all consistently associated with significant impaired survival, contrary to surgical resection, radiotherapy and cisplatin-based chemotherapy. No differences between use of IMRT or fixed field and applied as adjuvant or definitive irradiation were noticed. Age >60 at diagnosis reduced the risk of dying from cancer (HR 0.67, 95% CI 0.43, 1.04) but increased the competing risk regarding death from other causes (HR 2.42, 95% CI 0.94, 6.22), and age consequently failed to exert a significant impact on OS.
Our study has limitations. All analyses in NSCCUP including ours suffer from low case numbers especially in a single clinic demanding collection over years. Meanwhile, some changes in clinical practice may have occurred, and these changes may not be limited to those noticed, e.g. cisplatin-based PORCT already introduced in 2004. However, the treatment in cohort 2 is in line with recommendations according to guidelines and clinical evidence (7, 8, 25–28, 30–33). Some analyses suffer from impossibility to obtain pathologic data not included in the available reports, and molecular data including p16 status is missing especially in cohort 1. In addition, Epstein–Barr virus (EBV) was not assessed, as Germany is a low-endemic EBV and nasopharynx cancer region (34) and as EBV included in TNM staging of NSCCUP introduced in 2018 in the 8th TNM edition (35). Unfortunately, no FFPE blocks from cohort 1 were available for EBV detection. Because of the retrospective character of our analyses, and despite great effort to obtain data from printed documentation regarding risk factors, some missing data remained. This could have led to the impossibility in demonstrating any survival benefit of HPV-related NSCCUP in multivariate analyses as missing FFPE blocks in cohort 1 reduced the power to detect such differences substantially.
Strengths of the study are consistency respective to the studied population treated at a single center. According to the latest review, our monocentric study comprising 115 definitive NSCCUP-P out of 272 patients with initially occult primary lesion (Figure 1) is the largest single-institution cohort of NSCCUP-P analyzed so far regarding diagnostic procedures, decision-making, treatment regimens and outcome not limited to survival (26, 33). A strength is the internal validation of Pi by bootstrapping (24). Paying attention to the heterogeneity of NSCCUP-P, we present evidence for successful treatment stratification possibilities.
Conclusions
The sound approach recommended by ASCO and NCCN (7, 8) and implemented into our clinical routine along establishing of the MDTB yielded positive effects by choosing the right treatment for the right NSCCUP-P approaching more often the ultimate goal providing the right treatment for the right patient at the right time, all the time (36). ND and cisplatin-based PORCT, whenever advised due to ECE+ or bilateral malignancy-positive neck nodes, improve outcome in NSCCUP-P.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of the University Leipzig (votes 201-10-12072010 and 202-10-12072010). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conceptualization, GW. Methodology, GW. Validation, GW, MW, and TW. Formal analysis, GW, MW, TW, SW, and VZ. Investigation, GW, MW, RK, and TG. Resources, GW, TK, RK, FL, AD, SW, and VZ. Data curation, GW, MW, TW, and SF. Writing—original draft preparation, GW and MW. Writing—review and editing, all authors. Visualization, GW and MW. Supervision, GW, SW, AD, and FL. Project administration, GW. Funding acquisition, GW and AD. All authors contributed to the article and approved the submitted version.
Funding
The study was partly supported by the grants LIFE-006 B7 and LIFE-007 D9 of the Leipzig Research Center for Civilization Diseases (LIFE) funded by the European Union, the European Fund for Regional Development (EFRE), and the Free State of Saxony. The funding sources did not influence the design of the study, collection, interpretation and analysis of the data, the preparation of this report, or the decision to publish. The authors received support through covering the costs of open access publishing from the German Research Foundation (DFG) and the Open Science Project of the University of Leipzig within the program Open Science Publishing.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all patients participating in the investigation and their families. We especially thank Dr. Andreas Boehm and Franziska Jablonski for providing some clinical data of NSCCUP-P, and the entire technical staff, all nurses and physicians in the involved departments.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.682088/full#supplementary-material
References
1. Martin H, Morfit HM. Cervical Lymph Node Metastasis as the First Symptom of Cancer. Surg Gynecol Obstet (1944) 78:133–59.
2. Pavlidis N, Khaled H, Gaafar R. A Mini Review on Cancer of Unknown Primary Site: A Clinical Puzzle for the Oncologists. J Adv Res (2015) 6(3):375–82. doi: 10.1016/j.jare.2014.11.007
3. Pavlidis N, Pentheroudakis G. Cancer of Unknown Primary Site. Lancet (2012) 379:1428–35. doi: 10.1016/S0140-6736(11)61178-1
4. Martin H, Romieu C. The Diagnostic Significance of a “Lump in the Neck”. Postgrad Med (1952) 11:491–500. doi: 10.1080/00325481.1952.11694298
5. Patel SA, Parvathaneni A, Parvathaneni U, Houlton JJ, Karni RJ, Liao JJ, et al. Post-Operative Therapy Following Transoral Robotic Surgery for Unknown Primary Cancers of the Head and Neck. Oral Oncol (2017) 72:150–6. doi: 10.1016/j.oraloncology.2017.07.019
6. Patel SA, Magnuson JS, Holsinger FC, Karni RJ, Richmon JD, Gross ND, et al. Robotic Surgery for Primary Head and Neck Squamous Cell Carcinoma of Unknown Site. JAMA Otolaryngol Head Neck Surg (2013) 139(11):1203–11. doi: 10.1001/jamaoto.2013.5189
7. National Comprehensive Cancer Network. Nccn Clinical Practice Guidelines in Oncology: Head and Neck Cancers, Vol. V1. (2016).
8. Maghami E, Ismaila N, Alvarez A, Chernock R, Duvvuri U, Geiger J, et al. Diagnosis and Management of Squamous Cell Carcinoma of Unknown Primary in the Head and Neck: Asco Guideline. J Clin Onol (2020) 38(22):2570–96. doi: 10.1200/JCO.20.00275
9. Aro K, Bäck L, Mäkitie A, Tapiovaara L. An Evaluation of the Diagnostic Methods in Head and Neck Cancer of Unknown Primary Site. Acta Otolaryngol (2018) 138(10):930–6. doi: 10.1080/00016489.2018.1484567
10. Mani N, George MM, Nash L, Anwar B, Homer JJ. Role of 18-Fludeoxyglucose Positron Emission Tomography-Computed Tomography and Subsequent Panendoscopy in Head and Neck Squamous Cell Carcinoma of Unknown Primary. Laryngoscope (2016) 126(6):1354–8. doi: 10.1002/lary.25783
11. Winter MC, Haberkorn U, Kratochwil C. Stellenwert Der Nuklearmedizinischen Diagnostik Bei CUP-Syndrom [Importance of Nuclear Medicine Diagnostics in CUP Syndrome]. Radiologe (2014) 54(2):134–9. doi: 10.1007/s00117-013-2548-8
12. Oeser A, Gaebel J, Dietz A, Wiegand S, Oeltze-Jafra S. Information Architecture for a Patient-Specific Dashboard in Head and Neck Tumor Boards. Int J Comput Assist Radiol Surg (2018) 13(8):1283–90. doi: 10.1007/s11548-018-1741-7
13. Boehm A, Müller S, Pankau T, Straub G, Bohn S, Fuchs M, et al. Computergestützte Assistenz Zur Verbesserung Der Therapie-Planung in Der Kopf-Hals-Onkologie [Computer Assistance to Improve Therapy Planning for Head Neck Oncology]. Laryngorhinootol (2011) 90(12):732–8. doi: 10.1055/s-0031-1295410
14. Meier J, Boehm A, Kielhorn A, Dietz A, Bohn S, Neumuth T. Design and Evaluation of a Multimedia Electronic Patient Record “Oncoflow” With Clinical Workflow Assistance for Head and Neck Tumor Therapy. Int J Comput Assist Radiol Surg (2014) 9(6):949–65. doi: 10.1007/s11548-014-0988-x
15. Rades D, Kühnel G, Wildfang I, Börner AR, Schmoll HJ, Knapp W. Localised Disease in Cancer of Unknown Primary (CUP): The Value of Positron Emission Tomography (PET) for Individual Therapeutic Management. Ann Oncol (2001) 12(11):1605–9. doi: 10.1023/A:1013107732572
16. Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre JL, Greiner RH, et al. Postoperative Irradiation With or Without Concomitant Chemotherapy for Locally Advanced Head and Neck Cancer. N Engl J Med (2004) 350(19):1945–52. doi: 10.1056/NEJMoa032641
17. Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al. Radiation Therapy Oncology Group 9501/Intergroup. Postoperative Concurrent Radiotherapy and Chemotherapy for High-Risk Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med (2004) 350(19):1937–44. doi: 10.1056/NEJMoa032646
18. Wichmann G, Boehm A, Brus J, Horn IS, Gessner K, Kruber P, et al. Update on the Management of Advanced Head and Neck Cancer. Otorinolaringologia (2010) 60(2):65–80.
19. Schroeder L, Wichmann G, Willner M, Michel A, Wiesenfarth M, Flechtenmacher C, et al. Antibodies Against Human Papillomaviruses as Diagnostic and Prognostic Biomarker in Patients With Neck Squamous Cell Carcinoma From Unknown Primary Tumor. Int J Cancer (2018) 142(7):1361–8. doi: 10.1002/ijc.31167
20. Boehm A, Lindner F, Wichmann G, Bauer U, Wittekind C, Knoedler M, et al. Impact of Indication-Shift of Primary and Adjuvant Chemo Radiation in Advanced Laryngeal and Hypopharyngeal Squamous Cell Carcinoma. Eur Arch Otorhinolaryngol (2015) 272(8):2017–25. doi: 10.1007/s00405-014-3134-z
21. Kaplan EL, Meier P. Nonparametric Estimation From Incomplete Observations. J Am Stat Assoc (1958) 53:457. doi: 10.1080/01621459.1958.10501452
22. Mantel N. Evaluation of Survival Data and Two New Rank Order Statistics Arising in its Consideration. Cancer Chemother Rep (1966) 50:163–70.
23. Cox DR. Regression Models and Life-Tables. J R Stat Soc B (1972) 34:187–220. doi: 10.1111/j.2517-6161.1972.tb00899.x
24. Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD Statement. Ann Intern Med (2015) 162:55–63. doi: 10.7326/M14-0697
25. Johansen J, Buus S, Loft A, Keiding S, Overgaard M, Hansen HS, et al. Prospective Study of 18FDG-PET in the Detection and Management of Patients With Lymph Node Metastases to the Neck From an Unknown Primary Tumor. Results From the DAHANCA-13 Study. Head Neck (2008) 30(4):471–8. doi: 10.1002/hed.20734
26. Di Maio P, Iocca O, De Virgilio A, Ferreli F, Cristalli G, Pellini R, et al. Role of Palatine Tonsillectomy in the Diagnostic Workup of Head and Neck Squamous Cell Carcinoma of Unknown Primary Origin: A Systematic Review and Meta-Analysis. Head Neck (2019) 41(4):1112–21. doi: 10.1002/hed.25522
27. Cianchetti M, Mancuso AA, Amdur RJ, Werning JW, Kirwan J, Morris CG, et al. Diagnostic Evaluation of Squamous Cell Carcinoma Metastatic to Cervical Lymph Nodes From an Unknown Head and Neck Primary Site. Laryngoscope (2009) 119(12):2348–54. doi: 10.1002/lary.20638
28. Podeur P, Mancini J, Delgrande J, Santini L, Salas S, Wong S, et al. Role of Tonsillectomy in the Management of Carcinomas of Unknown Primary of the Head and Neck: A Retrospective Study Based on p16 Analysis. Front Oncol (2020) 10:594168. doi: 10.3389/fonc.2020.594168
29. Freitag J, Wald T, Kuhnt T, Gradistanac T, Kolb M, Dietz A, et al. Extracapsular Extension of Neck Nodes and Absence of Human Papillomavirus 16-DNA are Predictors of Impaired Survival in p16-positive Oropharyngeal Squamous Cell Carcinoma. Cancer (2020) 126(9):1856–72. doi: 10.1002/cncr.32667
30. Fakhrian K, Thamm R, Knapp S, Molls M, Pigorsch S, Haller B, et al. Radio(Chemo)Therapy in the Management of Squamous Cell Carcinoma of Cervical Lymph Nodes From an Unknown Primary Site. A Retrospective Analysis. Strahlenther Onkol (2012) 188(1):56–61. doi: 10.1007/s00066-011-0017-8
31. Sprave T, Rühle A, Hees K, Kalckreuth T, Verma V, Stoian, et al. Radiotherapeutic Management of Cervical Lymph Node Metastases From an Unknown Primary Site - Experiences From a Large Cohort Treated With Modern Radiation Techniques. Radiat Oncol (2020) 15(1):80. doi: 10.1186/s13014-020-01529-z
32. Chen AM, Farwell DG, Lau DH, Li BQ, Luu Q, Donald PJ. Radiation Therapy in the Management of Head-and-Neck Cancer of Unknown Primary Origin: How Does the Addition of Concurrent Chemotherapy Affect the Therapeutic Ratio? Int J Radiat Oncol Biol Phys (2011) 81(2):346–52. doi: 10.1016/j.ijrobp.2010.06.031
33. von der Grün J, Tahtali A, Ghanaati S, Rödel C, Balermpas P. Diagnostic and Treatment Modalities for Patients With Cervical Lymph Node Metastases of Unknown Primary Site – Current Status and Challenges. Radiat Oncol (2017) 12:82. doi: 10.1186/s13014-017-0817-9
34. Chang ET, Adami HO. The Enigmatic Epidemiology of Nasopharyngeal Carcinoma. Cancer Epidemiol Biomarkers Prev (2006) 15(10):1765–77. doi: 10.1158/1055-9965.EPI-06-0353
35. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge From a Population-Based to a More “Personalized” Approach to Cancer Staging. CA Cancer J Clin (2017) 67:93–9. doi: 10.3322/caac.21388
Keywords: cancer of unknown primary (CUP), neck dissection (ND), extracapsular extension of neck nodes (ECE), cisplatin-based postoperative radio-chemotherapy, outcome research, head and neck cancer (HNC), head and neck squamous cell carcinoma (HNSCC)
Citation: Wichmann G, Willner M, Kuhnt T, Kluge R, Gradistanac T, Wald T, Fest S, Lordick F, Dietz A, Wiegand S and Zebralla V (2021) Standardized Diagnostics Including PET-CT Imaging, Bilateral Tonsillectomy and Neck Dissection Followed by Risk-Adapted Post-Operative Treatment Favoring Radio-Chemotherapy Improve Survival of Neck Squamous Cell Carcinoma of Unknown Primary Patients. Front. Oncol. 11:682088. doi: 10.3389/fonc.2021.682088
Received: 17 March 2021; Accepted: 07 April 2021;
Published: 07 May 2021.
Edited by:
Panagiotis Balermpas, University Hospital Zürich, SwitzerlandReviewed by:
Jens Von Der Gruen, University Hospital Frankfurt, GermanyAlexander Rühle, Freiburg University Medical Center, Germany
Copyright © 2021 Wichmann, Willner, Kuhnt, Kluge, Gradistanac, Wald, Fest, Lordick, Dietz, Wiegand and Zebralla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gunnar Wichmann, Gunnar.Wichmann@medizin.uni-leipzig.de
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share senior authorship
 Gunnar Wichmann
Gunnar Wichmann Maria Willner
Maria Willner Thomas Kuhnt
Thomas Kuhnt Regine Kluge3
Regine Kluge3 Florian Lordick
Florian Lordick Andreas Dietz
Andreas Dietz Susanne Wiegand
Susanne Wiegand Veit Zebralla
Veit Zebralla