Abstract
Purpose
The role of maintenance immunotherapy with anti-CD20 monoclonal antibody rituximab in primary central nervous system lymphoma (PCNSL) is unclear. We retrospectively reviewed the medical records of all immunocompetent adults with newly diagnosed PCNSL treated at our institution between1996 and 2017.
Methods
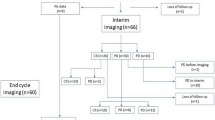
We identified 66 patients who attained complete response (CR) after completion of first-line regimen; 20 received maintenance therapy (maintenance therapy group) and 46 were observed with serial MRI scans without maintenance therapy (no-maintenance therapy group).
Results
Compared to the surveillance group, there was a significant increase in duration of survival (HR 0.27, 95% CI 0.08–0.98, P = 0.046) in the maintenance therapy group while the reduction in the risk of progression was not significant (HR: 0.61, 95% CI 0.26–1.43, P = 0.259).
Conclusion
We are evaluating the effectiveness of maintenance immunotherapy in PCNSL in a prospective multicenter randomized clinical trial.

Similar content being viewed by others
References
Abrey LE, Batchelor TT, Ferreri AJ et al (2005) (2005) Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 23(22):5034–5043
Grommes C, DeAngelis LM (2017) Primary CNS lymphoma. J Clin Oncol 35(21):2410–2418
Neuwelt EA, Schiff D (2015) Primary CNS lymphoma: a landmark trial and the next steps. Neurology 84(12):1194–1195
Ambady P, Holdhoff M, Bonekamp D et al (2015) Late relapses in primary CNS lymphoma after complete remissions with high-dose methotrexate monotherapy. CNS Oncol 4(6):393–398
Jahnke K, Thiel E, Martus P et al (2006) Relapse of primary central nervous system lymphoma: clinical features, outcome and prognostic factors. J Neurooncol 80(2):159–165
Hochster H, Weller E, Gascoyne RD et al (2009) Maintenance rituximab after cyclophosphamide, vincristine, and prednisone prolongs progression-free survival in advanced indolent lymphoma: results of the randomized phase III ECOG1496 Study. J Clin Oncol 27(10):1607–1614
Salles G, Seymour JF, Offner F et al (2011) Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet 377(9759):42–51
Sehn LH, Assouline SE, Stewart DA et al (2012) A phase 1 study of obinutuzumab induction followed by 2 years of maintenance in patients with relapsed CD20-positive B-cell malignancies. Blood 119(22):5118–5125
Ney DE, Abrey LE (2009) Maintenance therapy for central nervous system lymphoma with rituximab. Leuk Lymphoma 50(9):1548–1551
Angelov L, Doolittle ND, Kraemer DF et al (2009) Blood-brain barrier disruption and intra-arterial methotrexate-based therapy for newly diagnosed primary CNS lymphoma: a multi-institutional experience. J Clin Oncol 27(21): 3503–3509
Neuwelt EA, Goldman DL, Dahlborg SA et al (1991) Primary CNS lymphoma treated with osmotic blood-brain barrier disruption: prolonged survival and preservation of cognitive function. J Clin Oncol 9(9):1580–1590
Doolittle ND, Miner ME, Hall WA et al (2000) Safety and efficacy of a multicenter study using intraarterial chemotherapy in conjunction with osmotic opening of the blood-brain barrier for the treatment of patients with malignant brain tumors. Cancer 88(3):637–647
McAllister LD, Doolittle ND, Guastadisegni PE et al (2000) Cognitive outcomes and long-term follow-up results after enhanced chemotherapy delivery for primary central nervous system lymphoma. Neurosurgery 46(1):51–60 discussion 60–51
Holdhoff M, Ambady P, Abdelaziz A et al (2014) High-dose methotrexate with or without rituximab in newly diagnosed primary CNS lymphoma. Neurology 83(3):235–239
Ferreri AJ, Cwynarski K, Pulczynski E et al (2016) Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol 3(5):e217-227
Doolittle N, Kraemer D, Lacy C et al (2010) Enhanced delivery of rituximab in combination with methotrexate-based blood-brain barrier disruption for patients with newly diagnosed primary CNS lymphoma. Blood 116(21):2792
Bromberg JEC, Issa S, Bakunina K et al (2019) Rituximab in patients with primary CNS lymphoma (HOVON 105/ALLG NHL 24): a randomised, open-label, phase 3 intergroup study. Lancet Oncol 20(2):216–228
Rubenstein JL, Fridlyand J, Abrey L et al (2007) Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J Clin Oncol 25(11):1350–1356
Muller C, Murawski N, Wiesen MH et al (2012) The role of sex and weight on rituximab clearance and serum elimination half-life in elderly patients with DLBCL. Blood 119(14):3276–3284
Muldoon LL, Lewin SJ, Dosa E et al (2011) Imaging and therapy with rituximab anti-CD20 immunotherapy in an animal model of central nervous system lymphoma. Clin Cancer Res 17(8):2207–2215
Ambady P, Fu R, Netto JP et al (2017) Patterns of relapse in primary central nervous system lymphoma: inferences regarding the role of the neuro-vascular unit and monoclonal antibodies in treating occult CNS disease. Fluids Barriers CNS 14(1):16
Jahnke K, Hummel M, Korfel A et al (2006) Detection of subclinical systemic disease in primary CNS lymphoma by polymerase chain reaction of the rearranged immunoglobulin heavy-chain genes. J Clin Oncol 24(29):4754–4757
Acknowledgements
This work was supported in part by National Institute of Health Grants CA137488, NS044687, a Veterans Administration Merit Review Grant and the Walter S. and Lucienne Driskill Foundation, all to EAN.
Funding
The authors have no relevant financial disclosures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ambady, P., Fu, R., Szidonya, L. et al. Impact of maintenance rituximab on duration of response in primary central nervous system lymphoma. J Neurooncol 147, 171–176 (2020). https://doi.org/10.1007/s11060-020-03411-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-020-03411-0